
Model Building Report
This document lists the results for the homology modelling project "BIOSC 1540 - Project" submitted to SWISS-MODEL workspace on Nov. 15, 2024, 9:23 p.m..The submitted primary amino acid sequence is given in Table T1.
If you use any results in your research, please cite the relevant publications:
- Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede TSWISS-MODEL: homology modelling of protein structures and complexes.Nucleic Acids Res 46, W296-W303. (2018)
 29788355
29788355 10.1093/nar/gky427
10.1093/nar/gky427 - Bienert S, Waterhouse A, de Beer TAP, Tauriello G, Studer G, Bordoli L, Schwede TThe SWISS-MODEL Repository - new features and functionality.Nucleic Acids Res 45, D313-D319. (2017)
 27899672
27899672 10.1093/nar/gkw1132
10.1093/nar/gkw1132 - Studer G, Tauriello G, Bienert S, Biasini M, Johner N, Schwede TProMod3 - A versatile homology modelling toolbox.PLOS Comp Biol 17(1), e1008667. (2021)
 33507980
33507980 10.1371/journal.pcbi.1008667
10.1371/journal.pcbi.1008667 - Studer G, Rempfer C, Waterhouse AM, Gumienny R, Haas J, Schwede TQMEANDisCo - distance constraints applied on model quality estimation.Bioinformatics 36, 1765-1771. (2020)
 31697312
31697312 10.1093/bioinformatics/btz828
10.1093/bioinformatics/btz828 - Bertoni M, Kiefer F, Biasini M, Bordoli L, Schwede TModeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology.Scientific Reports 7. (2017)
 28874689
28874689 10.1038/s41598-017-09654-8
10.1038/s41598-017-09654-8
Results
The SWISS-MODEL template library (SMTL version 2024-11-14, PDB release 2024-11-08) was searched with BLAST (Camacho et al.) and HHblits (Steinegger et al.) for evolutionary related structures matching the target sequence in Table T1. For details on the template search, see Materials and Methods. Overall 689 templates were found (Table T2).
Models
The following models were built (see Materials and Methods "Model Building"):
Model #01 |
File | Built with | Oligo-State | Ligands | GMQE | QMEANDisCo Global |
|---|---|---|---|---|---|---|
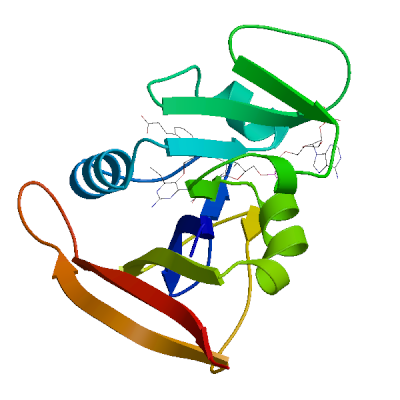
|
PDB | ProMod3 3.4.1 | monomer |
1 x MMV: 3-(2-{3-[(2,4-diamino-6-ethylpyrimidin-5-yl)oxy]propoxy}phenyl)propanoic acid;
1 x NAP: NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE; |
0.96 | 0.91 ± 0.07 |
|
|
| Template | Seq Identity | Oligo-state | QSQE | Found by | Method | Resolution | Seq Similarity | Range | Coverage | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 6e4e.1.A | 100.00 | monomer | 0.00 | HHblits | X-ray | 1.90Å | 0.62 | 1 - 159 | 1.00 | Dihydrofolate reductase |
Included Ligands
| Ligand | Description |
|---|---|
| 1 x MMV | 3-(2-{3-[(2,4-diamino-6-ethylpyrimidin-5-yl)oxy]propoxy}phenyl)propanoic acid |
| 1 x NAP | NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE |
Target MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
6e4e.1.A MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
Target EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
6e4e.1.A EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
Model #05 |
File | Built with | Oligo-State | Ligands | GMQE | QMEANDisCo Global |
|---|---|---|---|---|---|---|
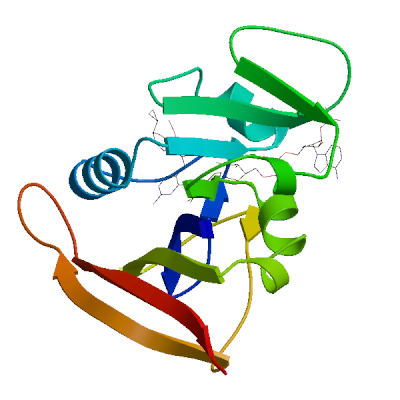
|
PDB | ProMod3 3.4.1 | monomer |
1 x NDP: NADPH DIHYDRO-NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE;
1 x XCF: 5-[[(2R)-2-cyclopropyl-7,8-dimethoxy-2H-chromen-5-yl]methyl]pyrimidine-2,4-diamine; |
0.95 | 0.91 ± 0.07 |
|
|
| Template | Seq Identity | Oligo-state | QSQE | Found by | Method | Resolution | Seq Similarity | Range | Coverage | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 3fyw.1.A | 100.00 | monomer | 0.00 | HHblits | X-ray | 2.10Å | 0.62 | 2 - 159 | 0.99 | Dihydrofolate reductase |
Included Ligands
| Ligand | Description |
|---|---|
| 1 x NDP | NADPH DIHYDRO-NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE |
| 1 x XCF | 5-[[(2R)-2-cyclopropyl-7,8-dimethoxy-2H-chromen-5-yl]methyl]pyrimidine-2,4-diamine |
Target MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
3fyw.1.A -TLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
Target EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
3fyw.1.A EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
Model #03 |
File | Built with | Oligo-State | Ligands | GMQE | QMEANDisCo Global |
|---|---|---|---|---|---|---|
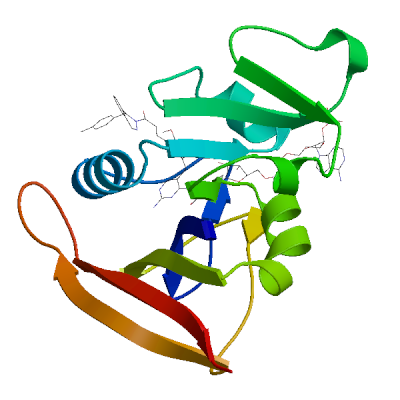
|
PDB | ProMod3 3.4.1 | monomer |
1 x NAP: NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE;
1 x OWS: (2E)-3-{5-[(2,4-diaminopyrimidin-5-yl)methyl]-2,3-dimethoxyphenyl}-1-[(1S)-1-(4-methylphenyl)phthalazin-2(1H)-yl]prop-2-en-1-one; |
0.95 | 0.91 ± 0.07 |
|
|
| Template | Seq Identity | Oligo-state | QSQE | Found by | Method | Resolution | Seq Similarity | Range | Coverage | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 6pr6.1.A | 100.00 | monomer | 0.00 | HHblits | X-ray | 2.01Å | 0.62 | 2 - 159 | 1.00 | Dihydrofolate reductase |
Included Ligands
| Ligand | Description |
|---|---|
| 1 x NAP | NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE |
| 1 x OWS | (2E)-3-{5-[(2,4-diaminopyrimidin-5-yl)methyl]-2,3-dimethoxyphenyl}-1-[(1S)-1-(4-methylphenyl)phthalazin-2(1H)-yl]prop-2-en-1-one |
Excluded ligands
| Ligand Name.Number | Reason for Exclusion | Description |
|---|---|---|
| GOL.3 | Not biologically relevant. | GLYCEROL |
| GOL.4 | Not biologically relevant. | GLYCEROL |
Target MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
6pr6.1.A MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
Target EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
6pr6.1.A EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
Model #06 |
File | Built with | Oligo-State | Ligands | GMQE | QMEANDisCo Global |
|---|---|---|---|---|---|---|
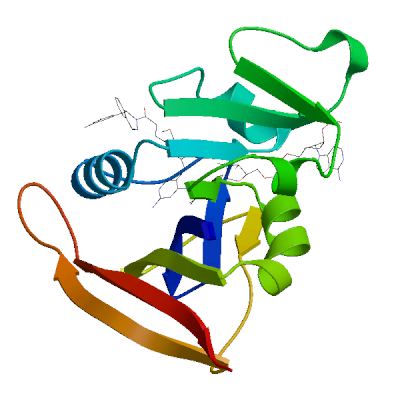
|
PDB | ProMod3 3.4.1 | monomer |
1 x NAP: NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE;
1 x OWJ: (2E)-3-{5-[(2,4-diaminopyrimidin-5-yl)methyl]-2,3-dimethoxyphenyl}-1-[(1S)-1-(3,5-dimethylphenyl)phthalazin-2(1H)-yl]prop-2-en-1-one; |
0.95 | 0.91 ± 0.07 |
|
|
| Template | Seq Identity | Oligo-state | QSQE | Found by | Method | Resolution | Seq Similarity | Range | Coverage | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 6pr8.1.A | 100.00 | monomer | 0.00 | HHblits | X-ray | 2.01Å | 0.62 | 2 - 159 | 0.99 | Dihydrofolate reductase |
Included Ligands
| Ligand | Description |
|---|---|
| 1 x NAP | NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE |
| 1 x OWJ | (2E)-3-{5-[(2,4-diaminopyrimidin-5-yl)methyl]-2,3-dimethoxyphenyl}-1-[(1S)-1-(3,5-dimethylphenyl)phthalazin-2(1H)-yl]prop-2-en-1-one |
Excluded ligands
| Ligand Name.Number | Reason for Exclusion | Description |
|---|---|---|
| GOL.3 | Not biologically relevant. | GLYCEROL |
| GOL.4 | Not biologically relevant. | GLYCEROL |
Target MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
6pr8.1.A -TLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
Target EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
6pr8.1.A EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
Model #04 |
File | Built with | Oligo-State | Ligands | GMQE | QMEANDisCo Global |
|---|---|---|---|---|---|---|
|
PDB | ProMod3 3.4.1 | monomer |
1 x NAP: NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE;
1 x Q11: 7-(2-methoxyphenyl)quinazoline-2,4-diamine; |
0.95 | 0.91 ± 0.07 |
|
|
| Template | Seq Identity | Oligo-state | QSQE | Found by | Method | Resolution | Seq Similarity | Range | Coverage | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 3sqy.1.A | 100.00 | monomer | 0.00 | HHblits | X-ray | 1.50Å | 0.62 | 2 - 158 | 1.00 | Dihydrofolate reductase |
Included Ligands
| Ligand | Description |
|---|---|
| 1 x NAP | NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE |
| 1 x Q11 | 7-(2-methoxyphenyl)quinazoline-2,4-diamine |
Target MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
3sqy.1.A MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
Target EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
3sqy.1.A EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
Model #07 |
File | Built with | Oligo-State | Ligands | GMQE | QMEANDisCo Global |
|---|---|---|---|---|---|---|
|
PDB | ProMod3 3.4.1 | monomer |
1 x NAP: NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE;
1 x Q12: 7-(3,4-dimethoxyphenyl)-6-methylquinazoline-2,4-diamine; |
0.95 | 0.91 ± 0.07 |
|
|
| Template | Seq Identity | Oligo-state | QSQE | Found by | Method | Resolution | Seq Similarity | Range | Coverage | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 3sr5.1.A | 100.00 | monomer | 0.00 | HHblits | X-ray | 1.68Å | 0.62 | 2 - 158 | 0.99 | Dihydrofolate reductase |
Included Ligands
| Ligand | Description |
|---|---|
| 1 x NAP | NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE |
| 1 x Q12 | 7-(3,4-dimethoxyphenyl)-6-methylquinazoline-2,4-diamine |
Target MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
3sr5.1.A -TLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
Target EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
3sr5.1.A EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
Model #08 |
File | Built with | Oligo-State | Ligands | GMQE | QMEANDisCo Global |
|---|---|---|---|---|---|---|
|
PDB | ProMod3 3.4.1 | monomer |
1 x NDP: NADPH DIHYDRO-NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE;
1 x TOP: TRIMETHOPRIM; |
0.95 | 0.91 ± 0.07 |
|
|
| Template | Seq Identity | Oligo-state | QSQE | Found by | Method | Resolution | Seq Similarity | Range | Coverage | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 2w9g.1.A | 100.00 | monomer | 0.00 | HHblits | X-ray | 1.95Å | 0.62 | 2 - 158 | 1.00 | DIHYDROFOLATE REDUCTASE |
Included Ligands
| Ligand | Description |
|---|---|
| 1 x NDP | NADPH DIHYDRO-NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE |
| 1 x TOP | TRIMETHOPRIM |
Target MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
2w9g.1.A MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
Target EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
2w9g.1.A EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
Model #09 |
File | Built with | Oligo-State | Ligands | GMQE | QMEANDisCo Global |
|---|---|---|---|---|---|---|
|
PDB | ProMod3 3.4.1 | monomer |
1 x TOP: TRIMETHOPRIM;
|
0.94 | 0.90 ± 0.07 |
|
|
| Template | Seq Identity | Oligo-state | QSQE | Found by | Method | Resolution | Seq Similarity | Range | Coverage | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 2w9h.1.A | 100.00 | monomer | 0.00 | HHblits | X-ray | 1.48Å | 0.62 | 2 - 158 | 1.00 | DIHYDROFOLATE REDUCTASE |
Included Ligands
| Ligand | Description |
|---|---|
| 1 x TOP | TRIMETHOPRIM |
Excluded ligands
| Ligand Name.Number | Reason for Exclusion | Description |
|---|---|---|
| EDO.1 | Not biologically relevant. | 1,2-ETHANEDIOL |
Target MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
2w9h.1.A MTLSILVAHDLQRVIGFENQLPWHLPNDLKHVKKLSTGHTLVMGRKTFESIGKPLPNRRNVVLTSDTSFNVEGVDVIHSI
Target EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
2w9h.1.A EDIYQLPGHVFIFGGQTLFEEMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
Model #02 |
File | Built with | Oligo-State | Ligands | GMQE | QMEANDisCo Global |
|---|---|---|---|---|---|---|
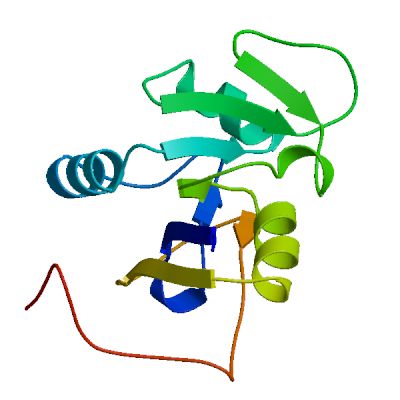
|
PDB | ProMod3 3.4.1 | monomer |
None
|
0.64 | 0.72 ± 0.07 |
|
|
| Template | Seq Identity | Oligo-state | QSQE | Found by | Method | Resolution | Seq Similarity | Range | Coverage | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 2w3m.1.A | 30.07 | monomer | 0.00 | BLAST | X-ray | 1.60Å | 0.37 | 2 - 144 | 0.90 | DIHYDROFOLATE REDUCTASE |
Excluded ligands
| Ligand Name.Number | Reason for Exclusion | Description |
|---|---|---|
| FOL.2 | Binding site not conserved. | FOLIC ACID |
| NDP.1 | Binding site not conserved. | NADPH DIHYDRO-NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE |
Target MTLSILVAHDLQRVIGFENQLPWH-LPNDLKHVKKLSTGHTL-------VMGRKTFESI---GKPLPNRRNVVLTSDTSF
2w3m.1.A -SLNCIVAVSQNMGIGKNGDLPWPPLRNEFRYFQRMTTTSSVEGKQNLVIMGKKTWFSIPEKNRPLKGRINLVLSRELKE
Target NVEGVDVI-HSIEDIYQLPGH---------VFIFGGQTLFEEMIDKVD--DMYITVIEGKFRGDTFFPPYTFEDWEVASS
2w3m.1.A PPQGAHFLSRSLDDALKLTEQPELANKVDMVWIVGGSSVYKEAMNHPGHLKLFVTRIMQDFESDTFFPEIDLEKYKLLPE
Target VEGKLDEKNTIPHTFLHLIRKK
2w3m.1.A YPGVLSD---------------
Materials and Methods
Template Search
Template search with BLAST and HHblits has been performed against the SWISS-MODEL template library (SMTL, last update: 2024-11-14, last included PDB release: 2024-11-08).
The target sequence was searched with BLAST against the primary amino acid sequence contained in the SMTL. A total of 337 templates were found.
An initial HHblits profile has been built using the procedure outlined in (Steinegger et al.), followed by 1 iteration of HHblits against Uniclust30 (Mirdita, von den Driesch et al.). The obtained profile has then be searched against all profiles of the SMTL. A total of 387 templates were found.
Template Selection
For each identified template, the template's quality has been predicted from features of the target-template alignment. The templates with the highest quality have then been selected for model building.
Model Building
Models are built based on the target-template alignment using ProMod3 (Studer et al.). Coordinates which are conserved between the target and the template are copied from the template to the model. Insertions and deletions are remodelled using a fragment library. Side chains are then rebuilt. Finally, the geometry of the resulting model is regularized by using a force field.
Model Quality Estimation
The global and per-residue model quality has been assessed using the QMEAN scoring function (Studer et al.).
Ligand Modelling
Ligands present in the template structure are transferred by homology to the model when the following criteria are met: (a) The ligands are annotated as biologically relevant in the template library, (b) the ligand is in contact with the model, (c) the ligand is not clashing with the protein, (d) the residues in contact with the ligand are conserved between the target and the template. If any of these four criteria is not satisfied, a certain ligand will not be included in the model. The model summary includes information on why and which ligand has not been included.
Oligomeric State Conservation
The quaternary structure annotation of the template is used to model the target sequence in its oligomeric form. The method (Bertoni et al.) is based on a supervised machine learning algorithm, Support Vector Machines (SVM), which combines interface conservation, structural clustering, and other template features to provide a quaternary structure quality estimate (QSQE). The QSQE score is a number between 0 and 1, reflecting the expected accuracy of the interchain contacts for a model built based a given alignment and template. Higher numbers indicate higher reliability. This complements the GMQE score which estimates the accuracy of the tertiary structure of the resulting model.
References
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TLBLAST+: architecture and applications.BMC Bioinformatics, 10, 421-430. (2009)
 20003500
20003500 10.1186/1471-2105-10-421
10.1186/1471-2105-10-421 - Steinegger M, Meier M, Mirdita M, Vöhringer H, Haunsberger SJ, Söding JHH-suite3 for fast remote homology detection and deep protein annotation.BMC Bioinformatics 20, 473. (2019)
 31521110
31521110 10.1186/s12859-019-3019-7
10.1186/s12859-019-3019-7 - Mirdita M, von den Driesch L, Galiez C, Martin MJ, Söding J, Steinegger MUniclust databases of clustered and deeply annotated protein sequences and alignments.Nucleic Acids Res, 45, D170–D176. (2016)
 27899574
27899574 10.1093/nar/gkw1081
10.1093/nar/gkw1081
Table T1:
Primary amino acid sequence for which templates were searched and models were built.
EMIDKVDDMYITVIEGKFRGDTFFPPYTFEDWEVASSVEGKLDEKNTIPHTFLHLIRKK
Table T2:
| Template | Seq Identity | Oligo-state | QSQE | Found by | Method | Resolution | Seq Similarity | Coverage | Description |
|---|---|---|---|---|---|---|---|---|---|
| 6e4e.1.A | 100.00 | monomer | - | HHblits | X-ray | 1.90Å | 0.62 | 1.00 | Dihydrofolate reductase |
| 6pr6.1.A | 100.00 | monomer | - | HHblits | X-ray | 2.01Å | 0.62 | 1.00 | Dihydrofolate reductase |
| 3sqy.1.A | 100.00 | monomer | - | HHblits | X-ray | 1.50Å | 0.62 | 1.00 | Dihydrofolate reductase |
| 3fyw.1.A | 100.00 | monomer | - | HHblits | X-ray | 2.10Å | 0.62 | 0.99 | Dihydrofolate reductase |
| 6pr8.1.A | 100.00 | monomer | - | HHblits | X-ray | 2.01Å | 0.62 | 0.99 | Dihydrofolate reductase |
| 3sr5.1.A | 100.00 | monomer | - | HHblits | X-ray | 1.68Å | 0.62 | 0.99 | Dihydrofolate reductase |
| 2w9g.1.A | 100.00 | monomer | - | HHblits | X-ray | 1.95Å | 0.62 | 1.00 | DIHYDROFOLATE REDUCTASE |
| Q6G9D5.1.A | 98.74 | monomer | - | AFDB search | AlphaFold v2 | NA | 0.61 | 1.00 | Dihydrofolate reductase |
| 2w9h.1.A | 100.00 | monomer | - | HHblits | X-ray | 1.48Å | 0.62 | 1.00 | DIHYDROFOLATE REDUCTASE |
| 2w9s.1.A | 81.01 | monomer | - | HHblits | X-ray | 1.80Å | 0.56 | 0.99 | DIHYDROFOLATE REDUCTASE TYPE 1 FROM TN4003 |
| 2w9s.1.A | 81.13 | monomer | - | BLAST | X-ray | 1.80Å | 0.56 | 1.00 | DIHYDROFOLATE REDUCTASE TYPE 1 FROM TN4003 |
| 2w9t.1.A | 81.13 | monomer | - | BLAST | X-ray | 2.35Å | 0.56 | 1.00 | DIHYDROFOLATE REDUCTASE TYPE 1 |
| 2w9t.1.A | 81.01 | monomer | - | HHblits | X-ray | 2.35Å | 0.56 | 0.99 | DIHYDROFOLATE REDUCTASE TYPE 1 |
| 5uio.4.A | 35.03 | monomer | - | HHblits | X-ray | 1.93Å | 0.39 | 0.99 | Dihydrofolate reductase |
| 5uio.4.A | 36.31 | monomer | - | BLAST | X-ray | 1.93Å | 0.39 | 0.99 | Dihydrofolate reductase |
| 5uio.1.A | 35.03 | monomer | - | HHblits | X-ray | 1.93Å | 0.39 | 0.99 | Dihydrofolate reductase |
| 5uio.1.A | 36.31 | monomer | - | BLAST | X-ray | 1.93Å | 0.39 | 0.99 | Dihydrofolate reductase |
| 5uii.1.A | 36.13 | monomer | - | BLAST | X-ray | 1.35Å | 0.39 | 0.97 | Dihydrofolate reductase |
| 5uih.1.A | 34.62 | monomer | - | HHblits | X-ray | 1.65Å | 0.39 | 0.98 | Dihydrofolate reductase |
| 2d0k.2.A | 36.54 | monomer | - | BLAST | X-ray | 1.90Å | 0.39 | 0.98 | Dihydrofolate reductase |
| 2d0k.1.A | 36.54 | monomer | - | BLAST | X-ray | 1.90Å | 0.39 | 0.98 | Dihydrofolate reductase |
| 6ddw.1.A | 26.62 | monomer | - | HHblits | X-ray | 1.40Å | 0.35 | 0.97 | Dihydrofolate reductase |
| 6vs5.1.A | 26.62 | monomer | - | HHblits | X-ray | 1.76Å | 0.35 | 0.97 | Dihydrofolate reductase |
| 8cq9.1.B | 26.62 | homo-dimer | - | HHblits | X-ray | 1.75Å | 0.35 | 0.97 | Dihydrofolate reductase |
| 4m2x.2.A | 26.80 | monomer | - | HHblits | X-ray | 2.26Å | 0.35 | 0.96 | Dihydrofolate reductase |
| 6vv6.2.A | 26.62 | monomer | - | HHblits | X-ray | 2.24Å | 0.35 | 0.97 | Dihydrofolate reductase |
| 6vvb.1.A | 26.62 | monomer | - | HHblits | X-ray | 1.45Å | 0.35 | 0.97 | Dihydrofolate reductase |
| 3f8y.1.A | 28.93 | monomer | - | HHblits | X-ray | 1.45Å | 0.35 | 1.00 | Dihydrofolate reductase |
| 8cop.1.B | 26.62 | homo-dimer | 0.00 | HHblits | X-ray | 1.80Å | 0.35 | 0.97 | Dihydrofolate reductase |
| 6dav.2.A | 28.30 | monomer | - | HHblits | X-ray | 1.55Å | 0.35 | 1.00 | Dihydrofolate reductase |
| 3gyf.1.A | 28.30 | monomer | - | HHblits | X-ray | 1.70Å | 0.35 | 1.00 | Dihydrofolate reductase |
| 3fs6.1.A | 28.30 | monomer | - | HHblits | X-ray | 1.23Å | 0.35 | 1.00 | Dihydrofolate reductase |
| 4m6l.1.A | 28.30 | monomer | - | HHblits | X-ray | 1.70Å | 0.35 | 1.00 | Dihydrofolate reductase |
| 8cox.1.B | 26.62 | homo-dimer | - | HHblits | X-ray | 2.10Å | 0.35 | 0.97 | Dihydrofolate reductase |
| 3l3r.1.A | 28.93 | monomer | - | HHblits | X-ray | 2.00Å | 0.35 | 1.00 | Dihydrofolate reductase |
| 3ghv.1.A | 28.93 | monomer | - | HHblits | X-ray | 1.30Å | 0.35 | 1.00 | Dihydrofolate reductase |
| 2w3m.1.A | 28.30 | monomer | - | HHblits | X-ray | 1.60Å | 0.35 | 1.00 | DIHYDROFOLATE REDUCTASE |
| 1j3k.1.A | 28.21 | monomer | - | HHblits | X-ray | 2.10Å | 0.35 | 0.98 | Bifunctional dihydrofolate reductase-thymidylate synthase |
| 1j3k.1.B | 28.21 | monomer | - | HHblits | X-ray | 2.10Å | 0.35 | 0.98 | Bifunctional dihydrofolate reductase-thymidylate synthase |
| 3dga.2.A | 28.21 | monomer | - | HHblits | X-ray | 2.70Å | 0.35 | 0.98 | Bifunctional dihydrofolate reductase-thymidylate synthase |
| 3dg8.1.A | 28.21 | monomer | - | HHblits | X-ray | 2.58Å | 0.35 | 0.98 | Bifunctional dihydrofolate reductase-thymidylate synthase |
| 1j3j.1.B | 28.21 | monomer | - | HHblits | X-ray | 2.30Å | 0.35 | 0.98 | Bifunctional dihydrofolate reductase-thymidylate synthase |
| 3dg8.2.A | 28.21 | monomer | - | HHblits | X-ray | 2.58Å | 0.35 | 0.98 | Bifunctional dihydrofolate reductase-thymidylate synthase |
| 1j3j.1.A | 28.21 | monomer | - | HHblits | X-ray | 2.30Å | 0.35 | 0.98 | Bifunctional dihydrofolate reductase-thymidylate synthase |
| 3dga.1.A | 28.21 | monomer | - | HHblits | X-ray | 2.70Å | 0.35 | 0.98 | Bifunctional dihydrofolate reductase-thymidylate synthase |
| 6dav.2.A | 30.07 | monomer | - | BLAST | X-ray | 1.55Å | 0.37 | 0.90 | Dihydrofolate reductase |
| 3f8y.1.A | 30.77 | monomer | - | BLAST | X-ray | 1.45Å | 0.37 | 0.90 | Dihydrofolate reductase |
| 3l3r.1.A | 30.77 | monomer | - | BLAST | X-ray | 2.00Å | 0.37 | 0.90 | Dihydrofolate reductase |
| 3ghv.1.A | 30.77 | monomer | - | BLAST | X-ray | 1.30Å | 0.37 | 0.90 | Dihydrofolate reductase |
| 2w3m.1.A | 30.07 | monomer | - | BLAST | X-ray | 1.60Å | 0.37 | 0.90 | DIHYDROFOLATE REDUCTASE |
The table above shows the top 50 filtered templates. A further 357 templates were found which were considered to be less suitable for modelling than the filtered list.
1ao8.1.A, 1bzf.1.A, 1cd2.1.A, 1cz3.1.A, 1d1g.1.B, 1ddr.1.A, 1ddr.1.B, 1dds.1.B, 1df7.1.A, 1dhf.1.A, 1dhf.1.B, 1dhi.1.A, 1dhi.1.B, 1dhj.1.A, 1dhj.1.B, 1dis.1.A, 1dlr.1.A, 1dls.1.A, 1dr1.2.B, 1dra.1.A, 1dra.1.B, 1drb.1.A, 1drb.1.B, 1drf.1.A, 1e26.1.A, 1hfp.1.A, 1hfq.1.A, 1ia2.1.A, 1ia2.2.A, 1j3i.1.A, 1j3i.1.B, 1j3j.1.A, 1j3j.1.B, 1j3k.1.A, 1j3k.1.B, 1juv.1.A, 1klk.1.A, 1kmv.1.A, 1lud.1.A, 1mvt.1.A, 1ohj.1.A, 1ohk.1.A, 1pd8.1.A, 1pd9.1.A, 1pdb.1.A, 1qzf.2.A, 1rb2.1.A, 1rf7.1.A, 1rg7.1.A, 1rh3.1.A, 1s3v.1.A, 1s3y.1.A, 1tdr.1.A, 1tdr.1.B, 1u70.1.A, 1u71.1.A, 1vdr.1.A, 1vdr.1.B, 1vj3.1.A, 1yho.1.A, 1zdr.1.A, 1zdr.2.A, 2azn.1.A, 2bl9.1.A, 2bla.1.A, 2blc.1.A, 2cig.1.A, 2d0k.1.A, 2d0k.2.A, 2drc.1.A, 2drc.1.B, 2fzh.1.A, 2g6v.1.A, 2g6v.1.B, 2gd9.1.A, 2h2q.1.B, 2hm9.1.A, 2hqp.1.A, 2hxv.1.A, 2inq.1.A, 2ith.1.A, 2jyb.1.A, 2kgk.1.A, 2l28.1.A, 2lf1.1.A, 2o7p.1.A, 2o7p.1.B, 2oip.1.A, 2p4g.1.A, 2qk8.1.A, 2w3v.1.A, 2xw7.1.A, 2zza.1.A, 3cd2.1.A, 3cl9.2.B, 3clb.1.A, 3clb.1.B, 3clb.2.A, 3d84.1.A, 3dat.1.A, 3dau.1.A, 3dfr.1.A, 3dg8.1.A, 3dg8.2.A, 3dga.1.A, 3dga.2.A, 3dl5.1.A, 3dl6.1.A, 3e0b.1.A, 3eig.1.A, 3f0u.1.A, 3f8z.1.A, 3f91.1.A, 3fl8.5.A, 3fl9.2.A, 3fs6.1.A, 3fy9.1.A, 3ghc.1.A, 3gyf.1.A, 3hj3.1.A, 3i3r.1.A, 3i8a.1.A, 3ia4.1.A, 3ia5.1.A, 3inv.1.B, 3irm.2.B, 3irn.1.A, 3irn.1.B, 3irn.2.A, 3irn.2.B, 3ix9.1.A, 3ix9.1.B, 3jtw.1.A, 3jvx.1.A, 3jw3.1.A, 3jw5.1.A, 3k45.1.A, 3k74.1.A, 3kfy.1.A, 3kgy.1.A, 3ky8.1.A, 3ky8.1.B, 3lg4.1.A, 3m08.1.A, 3m09.1.A, 3n0h.1.A, 3nrr.1.A, 3nxo.1.A, 3q1h.1.A, 3qfx.1.A, 3qg2.1.A, 3qg2.1.B, 3qgt.1.A, 3qgt.1.B, 3ql0.1.A, 3qls.1.A, 3qlw.1.A, 3qlx.2.A, 3r33.1.A, 3rg9.1.A, 3ro9.1.A, 3s7a.1.A, 3sai.2.A, 3sgy.1.A, 3tq8.1.A, 3um5.1.A, 3um5.1.B, 3vco.1.A, 3zpc.1.A, 3zpc.1.B, 3zpg.1.A, 4cd2.1.A, 4dpd.1.A, 4dpd.1.B, 4eck.1.A, 4eck.1.B, 4eig.1.A, 4eil.2.B, 4eil.4.A, 4eiz.1.A, 4g8z.1.A, 4g95.1.A, 4gh8.1.A, 4h95.1.A, 4h97.1.A, 4h98.1.A, 4ha7.1.A, 4ha7.1.B, 4ixe.1.A, 4ixf.1.A, 4ixg.1.A, 4kd7.2.A, 4keb.1.A, 4keb.2.A, 4kjk.1.A, 4kl9.1.A, 4km0.2.A, 4km2.1.A, 4km2.2.A, 4ky4.3.B, 4m2x.1.A, 4m2x.2.A, 4m2x.4.A, 4m6j.1.A, 4m6l.1.A, 4m7u.1.A, 4m7v.1.A, 4or7.1.A, 4osg.1.A, 4osg.2.A, 4osg.3.A, 4osg.4.A, 4p3q.1.A, 4p3r.1.A, 4p66.1.A, 4p68.1.A, 4pth.1.A, 4ptj.1.A, 4q67.1.A, 4q6a.1.A, 4qi9.1.A, 4qi9.2.A, 4qjz.1.A, 4qle.1.A, 4qlf.1.A, 4qlg.1.A, 4tu5.1.A, 4x5i.1.A, 4xe6.1.A, 5cc9.1.A, 5ccc.1.A, 5dxv.1.A, 5dxv.2.A, 5hf0.1.A, 5hi6.1.A, 5hi6.2.A, 5hsr.1.A, 5isp.1.A, 5isq.1.A, 5ist.1.A, 5ja3.1.B, 5scm.1.A, 5scp.1.A, 5sd2.1.A, 5sd7.1.A, 5u27.1.A, 5uih.1.A, 5uii.1.A, 5ujf.1.A, 5w3q.1.A, 5xux.1.A, 5xv0.1.A, 5xv0.1.B, 5xv0.2.A, 5xv2.1.A, 5xv5.1.A, 5xv5.1.B, 5z6k.1.A, 5z6l.1.A, 5z6m.1.A, 6a2k.1.B, 6a2m.1.B, 6a2n.1.B, 6a2o.1.A, 6a2o.1.B, 6a2p.1.B, 6cqa.1.A, 6cxk.1.A, 6cxm.1.A, 6cyv.1.A, 6ddp.1.A, 6dds.1.A, 6dds.2.A, 6ddw.1.A, 6drs.1.A, 6lhi.1.B, 6nnh.1.A, 6nnh.3.B, 6p8c.1.A, 6rul.1.A, 6rum.1.A, 6uwq.1.A, 6uww.1.A, 6vcj.1.A, 6vs5.1.A, 6vv6.2.A, 6vv7.2.A, 6vv8.1.A, 6vv9.2.A, 6vvb.1.A, 6wep.1.A, 6xg4.1.A, 6xg5.1.A, 7ctw.1.B, 7cty.1.A, 7ese.1.A, 7f3b.1.A, 7f3y.1.A, 7f3y.1.B, 7f3z.1.A, 7f3z.1.B, 7fgx.2.A, 7fgy.1.A, 7fgy.2.B, 7k62.1.A, 7k68.1.A, 7k6c.1.A, 7km7.1.A, 7km9.1.A, 7l9t.1.A, 7lrh.1.A, 7lrh.1.B, 7lrh.2.A, 7lrh.2.B, 7mqp.1.A, 7myl.1.A, 7mym.3.A, 7nae.1.A, 7r6g.1.A, 7rgj.1.B, 7tj3.1.A, 7xh2.1.A, 7xi7.1.A, 7zzx.1.A, 8a0n.1.A, 8a0z.1.A, 8a0z.2.A, 8cop.1.B, 8cox.1.B, 8cq9.1.B, 8cqa.1.A, 8cqa.1.B, 8crh.1.A, 8crh.1.B, 8dae.1.A, 8e4f.1.A, 8f09.1.A, 8f09.2.A, 8jfb.1.A, 8jfb.1.B, 8jfd.1.B, 8jsy.1.A, 8jsy.1.B, 8jsy.2.B, 8jsy.3.A, 8jsy.3.B, 8ssx.1.A, 8szd.1.A, 8sze.1.A, 8ta1.1.A, 8ucx.1.A, 8uvz.1.A, 8v9r.1.G, 8vz4.1.A, 8wgm.1.A, 8wgm.1.B, 9c87.1.G